Development and manufacturing control of Ibuprofen tablets 400mg
Ibuprofen tablets 400mg contain the active ingredient ibuprofen, which is (±)-2-(p – isobutylphenyl) propionic acid. This study is to summarize the development and manufacturing information on of this product, including preformulation study, formulation development, manufacturing process and quality control items. Keywords: Ibuprofen tablets, development, manufacturing, preformulation, quality control
1. Preformulation study
Ibuprofen tablets 400mg contain the active ingredient ibuprofen, which is (±)-2-(p – isobutylphenyl) propionic acid. The structural formula is represented below:
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). It works by reducing hormones that cause inflammation and pain in the body. Ibuprofen is used to reduce fever and treat pain or inflammation caused by many conditions such as headache, toothache, back pain, arthritis, menstrual cramps, or minor injury. It has been a non-prescription drug since 1984.
1.1 Physical and chemical characteristics
Ibuprofen is very slightly soluble in water (< 1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. It has poor flow and compaction characteristics owing to its needle-like (acicular) crystalline structure and viscoelastic properties. The key physical and chemical characteristics are listed as below.
• Empirical Formula: C13H18O2
• Formula weight: 206.12gram/mole
• Appearance: White Powder
• Melting Point: 77-78 ℃
• Enthalpy formulation: – 14114 kJ/mol
• Solubility: Stable. Combustible. Incompatible with strong oxidizing agents
• Storage Condition: -20-25 ℃ (68-77 F)
1.2 Mechanism of action
Ibuprofen is a nonsteroidal anti-inflammatory drug, or NSAID. The term nonsteroidal indicates that ibuprofen is not a steroid, a common class of drugs used to reduce inflammation and swelling. Scientists believe that ibuprofen works by inhibiting cyclooxygenase (COX) which converts arachidonic acid to prostaglandins. Arachidonic acid is a naturally occurring fatty acid that is used to build a number of important biochemical compounds, including the prostaglandins. The prostaglandins are involved in the transmission of pain impulses throughout the nervous system. If the COX enzyme is prevented from functioning, arachidonic acid cannot be converted into prostaglandins and pain messages will not be transmitted.
Ibuprofen is especially effective in treating certain kinds of pain and inflammation, including those associated with menstrual cramps, various kinds of arthritis, headaches and migraines, pain from injuries and surgery, and discomfort associated with influenza and gout.
1.3 Indication and Usage
Carefully consider the potential benefits and risks of Ibuprofen tablets and other treatment options before deciding to use Ibuprofen tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. ? Ibuprofen tablets are indicated for relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis. ? Ibuprofen tablets are indicated for relief of mild to moderate pain. ? Ibuprofen tablets are also indicated for the treatment of primary dysmenorrhea. Controlled clinical trials to establish the safety and effectiveness of IBUPROFEN tablets in children have not been conducted.
1.4 ADME
Ibuprofen is rapidly absorbed after oral admin, & peak concns in plasma are observed after 15-30 min. The half-life in plasma is about 2 hr. Ibuprofen is extensively (99%) bound to plasma proteins, but the drug occupies only a fraction of the total drug-binding sites at usual concns. Ibuprofen passes slowly into the synovial spaces & may remain there in higher concn as the concns in plasma decline. In experimental animals, ibuprofen & its metabolites pass easily across the placenta. The excretion of ibuprofen is rapid & complete. More than 90% of an ingested dose is excreted in the urine as metabolites or their conjugates.
1.5 Side effects
The most frequent type of adverse reaction occurring with Ibuprofen tablets is gastrointestinal. In controlled clinical trials the percentage of patients reporting one or more gastrointestinal complaints ranged from 4% to 16%. In controlled studies when Ibuprofen tablets were compared to aspirin and indomethacin in equally effective doses, the overall incidence of gastrointestinal complaints was about half that seen in either the aspirin- or indomethacin-treated patients. Adverse reactions observed during controlled clinical trials at an incidence greater than 1% are listed in the table. Those reactions listed in Column one encompasses observations in approximately 3,000 patients. More than 500 of these patients were treated for periods of at least 54 weeks. Still other reactions occurring less frequently than 1 in 100 were reported in controlled clinical trials and from marketing experience. These reactions have been divided into two categories: Column two of the table lists reactions with therapy with Ibuprofen tablets where the probability of a causal relationship exists: for the reactions in Column three, a causal relationship with Ibuprofen tablets has not been established. Reported side effects were higher at doses of 3200 mg/day than at doses of 2400 mg or less per day in clinical trials of patients with rheumatoid arthritis. The increases in incidence were slight and still within the ranges reported in the table.
2. Formulation development
2.1 Protype formulation The product is developed based on the protype formulation as showed in Table 1.
Table 1- Protype Formulation Table
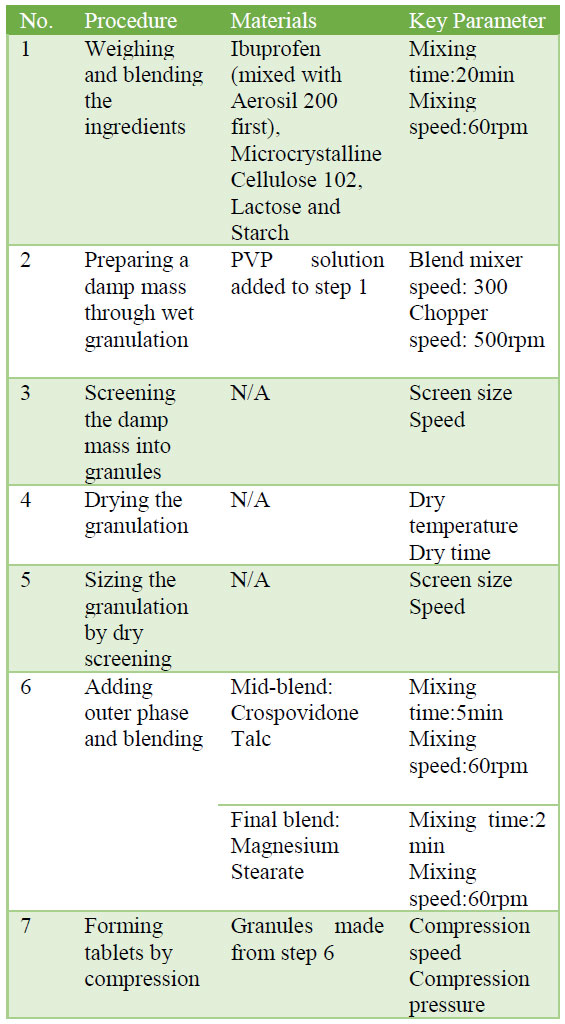
2.2 Formulation procedure
Wet granulation process is employed to produce the target product as following procedure in the lab:
1) Weight materials on weighing balance and pre-blend the materials: Ibuprofen (mixed with Aerosil 200 first), Microcrystalline Cellulose 102, Lactose and Starch
2) Transfer the powder blend to a shear granulator
3) Mix the material in the granulator for 2 minutes.
4) Prepare granulating solution by dissolving Poly vinyl pyrrolidone in 60 ml of water.
5) Open and transfer granulating solution Povidone solution into the granulator and mix for 2 minutes.
6) Open and check the granules to see granulation end point.
7) If granulation end point is not achieved, add additional water sufficient to achieve granulation end point.
8) Pass the wet mass through # 10 mesh hand screen.
9) Spread the wet mass carefully on an Aluminum tray and place in the hot air oven.
10) Set the inlet temperature to 95°C and dry until wet mass is completely dried.
11) Pass the dried granules through # 20 mesh screen and transfer into polybag.
12) Add Crospovidone to poly bag (Step 11); screen Talc through #40 mesh screen and transfer to poly bag (Step 11).
13) Manually mix the material in a polybag for 2 minutes.
14) Screen Magnesium Stearate through #40 mesh screen and transfer to poly bag (Step 13).
15) Manually mix the material in a polybag for 2 minutes.
16) Compress the tablet in compression machine
2.3 Problems encountered during formulation development
Capping is found during the compression process for granules. Capping happens when the upper or lower segment of the tablet separates horizontally, either partially or completely from the main body of a tablet and comes off as a cap, during ejection from the tablet press, or during subsequent handling. It indicated that maybe the protype formulation of granule is not so optimal and thus caused the capping issue and poor hardness as well. In this formulation, Ibuprofen is prescribed in high doses 400 mg while the whole weight for tablet is 700mg. As discussed in the part of “reformulation study”, Ibuprofen has poor flow and compaction characteristics owing to its needle-like (acicular) crystalline structure and viscoelastic properties. It gives much challenge of the formulation design to compress the granule into suitable-sized tablets. There are two basic ideas to improve the compact ability of the granules:
Use Modified crystal forms of Ibuprofen
Modified crystal forms of ibuprofen with optimized processing characteristics could be achieved by solvent evaporation and solvent change techniques in the presence of some additives (e.g., gelatin, Aerosil, and phosphate buffer). These methods are relatively simple and inexpensive. The modified crystals obtained were larger, rounded in appearance, noncohesive, exhibited better flow characteristics, and had higher density and compressibility compared with the common form. The resultant modifications exhibited an isomorphic nature with similar dissolution patterns. Tablets prepared from untreated raw ibuprofen showed minimal crushing strength and 100% capping tendency. In contrast, tablets prepared from the powder modifications showed higher crushing strengths and lower capping tendencies. The modified ibuprofen particles have potential application in tableting by direct compression as well as for filling in hard gelatin capsules.
Optimize the formulation of granules
The quantity of binder (PVP) could be improved to make the binding properties of granule better.
And the final mixing time with Lubricant (Magnesium Stearate) could be reduced to avoid excessive lubrication of granulation.
The final formulation is adjusted as showed in Table 2.
Table 2- Optimized Formulation Table
2.4 Scale-up batch and problems encountered
2.4.1 Scale-up
Scale-up is generally defined as the process of increasing batch size. Scale-up of a process can also be viewed as a procedure for applying the same process to different output volumes. Mainly there have three stages of process scales up batches: a) Trial Batches b) Exhibit Batches c) Validation Batches. After validation batches, large scale up of Ibuprofen tablets could be produced. The Chart below describes the step of process Scale up of Ibuprofen tablets.
Figure 1: Scale-up process of Ibuprofen tablets
2.4.2 Problems encountered
During scaling up, tableting process is considered as key step during the entire development. In the scale up stages, the capping issue during taletting is carefully avoided through the adjustment on the tableting machine.
Capping is usually due to the air–entrapment in a compact during compression, and subsequent expansion of tablet on ejection of a tablet from a die. Thus, from equipment viewpoint, the following actions could be used:
1) Polish dies properly. Investigate other steels or other materials.
2) Use flat punches.
3) Make proper setting of lower punch during ejection.
4) Adjust sweep-off blade correctly to facilitate proper ejection.
5) Reduce speed of turret (Increase dwell time).
2.5 Packaging material selection
Blister packs are commonly used as unit-dose packaging for pharmaceutical tablets. Blister packs can provide barrier protection for shelf life requirements, and a degree of resistance. So, Ibuprofen tablets are to be packaged into blister. As Ibuprofen is normally stable, packaging materials used for the tablet stability study are selected as polyvinyl chloride (PVC) and aluminium foil.
3. Manufacturing Batch
3.1 Master formula
The master formula of Ibuprofen tablets 400mg is presented in Table 3, based on the batch size as 350kg.
Table 3- Master formula of Ibuprofen tablets 400mg
3.2 Safety precautions to be taken during manufacturing
Between dust and exposure to chemicals, the production processes at the facilities might pose dangers to worker health. Thus, personal protective equipment (PPE) that help protect workers during the creation of the product are important from the perspective of healthy human living. The PPEs such as disposable to reusable respirators, versatile powered and supplied air respiratory systems could be used in the manufacturing of Ibuprofen tablets.
3.3 Equipment Used
3.3.1 Granulation process
The key equipment for the manufacturing of Ibuprofen granules are listed in the table 4 below:
Table 4- Key equipment for granulation process
3.3.1 Tableting process
A few types of tableting machines could be used for the compression process of Ibuprofen tablets, such as Fette 2090i tablet press, GEA Courtoy Rotary Tablet Presses.
3.4 Compounding procedure
The Compounding procedure required for the manufacturing of Ibuprofen tablets are briefly summarized in Table 5.
Table 5- Compounding procedure of Ibuprofen tablets 400mg
3.5 Packaging materials, packaging equipment and procedure
The stability study for Ibuprofen tablets 400mg show that the packaging materials of polyvinyl chloride (PVC) and aluminium foil are suitable for the targeted shelf life. The types below of Blister
Packing Machines could be used for the packaging process:
• Rotary thermoforming machine-BP 102, Cartoblis, BP 450AD machines from ACG Pampac operate on this principle
• Flat forming and rotary sealing-B 45, Unipac machines from ACG Pampac operate on this principle
• Flat forming & flat sealing machine-BQS & Miniblis machines from ACG Pampac operate on this principle
The produce of packaging involves:
• Heating the plastic ? Plastic thermoforming into blister cavities
• Loading the blister with the product ? Placing lidding material over the blister
• Heat-sealing the package
4. Quality control
4.1 IPC (in-process-control) test
IPC test for granulation process and tableting process are listed as tables below:
Table 6- IPC test for granulation process
Table 7- IPC test for tableting process
4.2 QC release test
The analysis test items of QC release test are listed in Table below, with the brief summary on specification and test purpose.
Table 8- QC release test
References
[1] Improving the Physical and Chemical Properties of Ibuprofen. Aly Nada. Pharmaceutical Technology, 2005, Volume 29, Issue 11
[2] Formulation development and optimization of Ibuprofen tablets by direct compression method.Bushra R.Pak J Pharm Sci. 2008, 21(2):113-20.
[3] Process Scale Up Of Ibrufen Tablet. Shashank Tiwari et al /J. Pharm. Sci. & Res. Vol.3 (10), 2011, 1525-1529
[4] Manufacturing Defects Of Tablets – A Review. Abhinav Singh Rana. Journal of Drug Delivery & Therapeutics; 2013, 3(6), 200-206
[5] USP 36- monograph, Ibuprofen tablets
[6] Handbook of Pharmaceutical Excipients, sixth edition. Raymond C Rowe, etc.
[7] Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, ninth edition. Howard C. Ansel, etc.
[8] Pubchem datatbase. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3672
[9] Date base. http://ww.Rxlist.com
We make good
balance for quality,
cost, and efficiency